Abstract
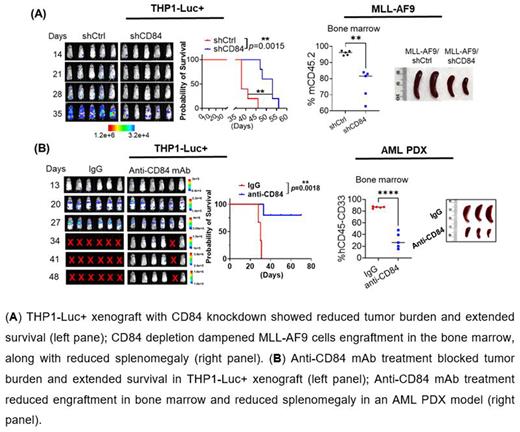
CD84 is a member of the signaling lymphocyte activation molecule (SLAM) family; it forms homophilic dimers by self-association. CD84 is reported as an important survival receptor in chronic lymphocytic leukemia and its activation upregulates PD-L1 expression on leukemia cells. We and others recently reported that CD84 is highly expressed on myeloid derived suppressor cells, but its function in myeloid malignancies has yet to be investigated. Using publicly available microarray data (GSE13509), we found that CD84 is overexpressed on acute myeloid leukemia (AML) cells compared to normal hematopoietic counterparts (AML, n=542 vs. normal, n=73, p<0.0001). and that its upregulation is associated with poor prognosis in AML patients (GSE10358; p=0.0105). Knocking down CD84 expression in AML cell lines (THP1 and HEL) by shRNAs caused a substantial inhibition of cell viability and extended THP1-luciferase xenografted mice survival in vivo (median survival: shCtrl, 38 days vs shCD84, 53 days, p=0.0015, n=5). Moreover, we observed that CD84 depletion in AML primary patient cells dramatically induced cell apoptosis (shCtrl: 13.0±1.84% vs shCD84: 29.33±1.3%; n=3; p=0.02) and inhibited colony formation (shCtrl: 68.44±11.65 vs shCD84: 15.56±6.32; n=3; p=0.01).
In light of highly expressed CD84 in MLL-r AML and inv(16) AML, we employed MLL-AF9 and CBFB-MYH11(CM) AML mouse models, and found that CD84 knockdown robustly dampened cell growth and decreased colony-forming units in vitro. In both models, bone marrow (BM) transplantation shown that CD84 deficiency significantly decreased leukemic engraftment in BM (MLL-AF9: shCtrl: 95.92±0.61% vs shCD84: 76.42±4.11%, p=0.0016, n=5/group; inv(16): shCtrl: 39.02±2.98% vs shCD84: 13.66±1.24%, p<0.0001, n=5/group), spleen, and peripheral blood (PB). In AML patient derived xenografts (PDX), we observed AML burden was markedly reduced in BM (shCtrl:51.22±10.29% vs shCD84: 2.304±0.16%; n=5/group, p=0.0014) and spleen (shCtrl: 8.790±2.989% vs shCD84: 0.8420±0.13%; n=5/group, p=0.0255) in the CD84 knockdown group.
Mechanistically, CD84 deletion depressed mTOR signaling, induced mitochondrial stress, and disrupted energy supply chains based on RNA-seq analysis. We demonstrated CD84 deletion caused mitochondrial dysfunction as indicated by decreasing oxidation phosphorylation (shCtrl vs shCD84, p<0.0001) and fatty acid oxidation (shCtrl vs shCD84, p=0.0004). Next, we determined that CD84 deletion caused disruption of mitochondrial matrix morphology and loss of mitochondrial cristae in AML. We also observed a dramatic loss of mitochondrial membrane potential upon CD84 deletion.
We generated a novel murine-human chimeric anti-human CD84 monoclonal antibody (mAb) with high affinity and specificity. In the presence of NK cells, anti-CD84 mAb induced strong antibody-dependent cell-mediated cytotoxicity in CD84-expressing AML lines (n=2) and primary samples (n=2), but not in healthy donor CD34+ cells (n=2). Next, the luciferase-expressing THP1 was transplanted into NSG mice. Mice with comparable tumor burden were randomized into 2 groups: control human IgG or anti-CD84 mAb (5mg/kg, i.v, bid, n=6/group), every week together with 3x106 human PB mononuclear cells (PBMCs) as effector cells (for 4 weeks). Anti-CD84 mAb significantly suppressed leukemia development and extended median survival (IgG: 30 days vs anti-CD84 mAb: undefined, p=0.0018). However, without PBMCs, human IgG or anti-CD84 mAb did not induce significant differences in tumor burden or median survival (n=5/group; p=0.5082).
Finally, we developed two AML PDXs in NSG mice. Following engraftment >1% in PB, mice were divided into 2 groups (n=6/group). We maintained the same treatment strategy as applied above. In both AML PDXs, the BM engraftment of CD45+CD33+ cells markedly decreased in anti-CD84 mAb treated mice (AML #1: IgG: 94.25± 0.41% vs anti-CD84 mAb: 71.52±3.96%, p= 0.0002; AML #2: IgG: 88.74±0.7% vs anti-CD84 mAb: 53.98±5.61%, p=0.0003). Anti-CD84mAb treatments in secondary transplantation of BM harvested from the first treatment groups resulted in further significant reduction in BM engraftment (IgG: 86.48± 0.67% vs anti-CD84 mAb: 29.74±6.02%, n=5, p<0.0001), and extended survival (IgG, 28 days vs anti-CD84 mAbs, 36 days, p=0.0026, n=5) following third BM transplantation. The results indicated our pharmacological targeting of CD84 inhibits leukemogenesis.
Disclosures
Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Lynx: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal